Fentenal Patch
Fentanyl injection is used to produce anesthesia for surgery and to treat pain before, during and after surgery. Includes fentanyl side effects, interactions and.
Consumer information about the medication FENTANYL - TRANSDERMAL Duragesic, includes side effects, drug interactions, recommended dosages, and storage information.
Nov 14, 2014 Fentanyl is available as a skin patch, lozenge, pills, shots, a film that dissolves in your mouth, nasal spray, or by IV intravenous. Use fentanyl.
Fentanyl patches may cause serious or life-threatening breathing problems, especially during the first 72 hours of your treatment and any time your dose is increased. Your doctor will monitor you carefully during your treatment. Because of this serious risk, fentanyl patches should only be used to treat people who are tolerant used to the effects of the medication to opioid medications because they have taken this type of medication for at least one week and should not be used to treat mild or moderate pain, short-term pain, pain after an operation or medical or dental procedure, or pain that can be controlled by medication that is taken as needed. Tell your doctor if you have or have ever had slowed breathing or asthma. Your doctor will probably tell you not to use fentanyl patches. Also tell your doctor if you have or have ever had lung disease such as chronic obstructive pulmonary disease COPD; a group of diseases that affect the lungs and airways, a head injury, or any condition that increases the amount of pressure in your brain. The risk that you will develop breathing problems may be higher if you are an older adult or are weak or malnourished due to disease. If you experience any of the following symptoms, call your doctor immediately or get emergency medical treatment: slowed breathing, long pauses between breaths, or shortness of breath.
Taking certain medications with fentanyl may increase the risk of serious or life-threatening breathing problems. Tell your doctor and pharmacist if you are taking or plan to take any of the following medications: amiodarone Cordarone, Pacerone ; aprepitant Emend ; carbamazepine Carbatrol, Epitol, Equetro, Tegretol, Teril ; certain antifungals such as fluconazole Diflucan, itraconazole Onmel, Sporanox, and ketoconazole Nizoral ; clarithromycin Biaxin, in Prevpac ; diltiazem Cardizem, Cartia, Dilacor, Dilt-CD, Diltzac, Taztia ; erythromycin E-Mycin, Erythrocin ; fosamprenavir Lexiva ; nefazodone; nelfinavir Viracept ; phenytoin Dilantin, Phenytek ; rifampin Rifadin, Rimactane, in Rifamate ; ritonavir Norvir, in Kaletra ; troleandomycin TAO not available in the United States ; and verapamil Calan, Covera, Verelan. Your doctor may need to change the dosages of your medications and will monitor you carefully.
Fentanyl patches may be habit-forming. Do not apply more patches, apply the patches more often, or use the patches in a different way than prescribed by your doctor. Tell your doctor if you or anyone in your family drinks or has ever drunk large amounts of alcohol; uses or has overused prescription medications; uses or has ever used street drugs; or has or has ever had depression or another mental illness. There is a greater risk that you will overuse fentanyl patches if you have or have ever had any of these conditions.
Do not allow anyone else to use your medication. Fentanyl patches may harm or cause death to other adults and children who use them. Store fentanyl patches in a safe place so that no one else can use them accidentally or on purpose. Be especially careful to keep fentanyl patches out of the reach of children. Keep track of how many patches are left so you will know if any are missing.
People who are not being treated with fentanyl patches may be seriously harmed or may die if the sticky side of a patch touches their skin. Be careful not to allow the sticky side of the patch to touch anyone else s skin. If you are holding or caring for children, make sure that they do not touch your patch. If the patch accidentally comes off of your body and sticks to another person s skin, immediately remove the patch, wash the area with clear water, and get emergency medical attention.
Fentanyl patches that have been worn for 3 days still contain enough medication to cause serious harm or death to adults or children who are not being treated with the medication. Never throw used or unused patches in a trash can or leave them in a place where they may be found by others, especially children. Dispose of used and unwanted patches properly according to instructions. See STORAGE and DISPOSAL.
If your fentanyl patch is exposed to extreme heat, it may release too much medication into your body at once. This can cause serious or life-threatening symptoms. Do not expose your patch or the skin around it to direct heat such as heating pads, electric blankets, heat lamps, saunas, hot tubs, and heated water beds. Do not take long, hot baths or sunbathe while you are wearing the patch. Your patch may also release too much medication if you have a fever or if you get very hot after physical activity. Avoid physical activity that might cause you to get very hot. Call your doctor right away if you have a fever. Your doctor may need to adjust your dose.
Tell your doctor if you are pregnant or plan to become pregnant. If you use fentanyl patches regularly during your pregnancy, your baby may experience life-threatening withdrawal symptoms after birth. Tell your baby s doctor right away if your baby experiences any of the following symptoms: irritability, hyperactivity, abnormal sleep, high-pitched cry, uncontrollable shaking of a part of the body, vomiting, diarrhea, or failure to gain weight.
Your doctor or pharmacist will give you the manufacturer s patient information sheet Medication Guide when you begin treatment with fentanyl patches and each time you fill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration FDA website or the manufacturer s website to obtain the Medication Guide.
Talk to your doctor about the risks of using this medication.
Fentanyl patches are used to relieve severe pain in people who are expected to need pain medication around the clock for a long time and who cannot be treated with other medications. Fentanyl is in a class of medications called opiate narcotic analgesics. It works by changing the way the brain and nervous system respond to pain.
Transdermal fentanyl comes as a patch to apply to the skin. The patch is usually applied to the skin once every 72 hours. Change your patch at about the same time of day every time you change it. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Apply fentanyl patches exactly as directed.
Your doctor may start you on a low dose fentanyl patch and gradually increase your dose, not more often than once every 3 days at first, and then not more often than once every 6 days. Your doctor may decrease your dose if you experience side effects. Talk to your doctor about how you are feeling during your treatment with fentanyl patches.
Fentanyl patches are only for use on the skin. Do not place patches in your mouth or chew or swallow the patches.
Do not stop using fentanyl patches without talking to your doctor. Your doctor will probably decrease your dose gradually. If you suddenly stop using fentanyl patches you may have symptoms of withdrawal. Call your doctor if you experience any of these symptoms of withdrawal: restlessness, teary eyes, runny nose, yawning, sweating, chills, muscle pain, large pupils black circles in the center of the eyes, irritability, anxiety, backache, pain in the joints, weakness, stomach cramps, difficulty falling asleep or staying asleep, nausea, loss of appetite, vomiting, diarrhea, fast heartbeat, or rapid breathing.
Do not use a fentanyl patch that is cut, damaged, or changed in any way. If you use cut or damaged patches, you may receive most or all of the medication at once, instead of slowly over 3 days. This may cause serious problems, including overdose and death.
You may bathe, swim, or shower while you are wearing a fentanyl patch. If the patch falls off during these activities, dispose of it properly. Then dry your skin completely and apply a new patch. Leave the new patch in place for 72 hours after you apply it.
You can apply a fentanyl patch to your chest, back, upper arms, or the sides of your waist. If you are applying the patch to a child or to a person who is unable to think clearly, choose an area on the upper back to make it more difficult for the person to remove the patch and place it in his or her mouth. Choose an area of skin that is flat and hairless. Do not apply the patch to parts of the body that move a lot or to skin that has been exposed to radiation or that is sensitive, very oily, broken out, irritated, broken, cut or damaged. If there is hair on the skin, use scissors to clip the hair as close to the skin as possible. Do not shave the area.
To apply the patch, follow these steps:
Clean the area where you plan to apply the patch with clear water and pat completely dry. Do not use any soaps, lotions, alcohols, or oils.
Tear open the pouch containing the fentanyl patch along the dotted line, starting at the slit. Remove the patch from the pouch and peel off both parts of the protective liner from the back of the patch. Try not to touch the sticky side of the patch.
Immediately press the sticky side of the patch onto the chosen area of skin with the palm of your hand.
Press the patch firmly for at least 30 seconds. Be sure that the patch sticks well to your skin, especially around the edges.
If the patch does not stick well or comes loose after it is applied, tape the edges to your skin with first aid tape. If the patch still does not stick well, you may cover it with Bioclusive or Tegaderm brand see-through dressings. Do not cover the patch with any other type of bandage or tape.
If a patch falls off before it is time to remove it, dispose of the patch properly and apply a new patch. Leave the new patch in place for 72 hours.
When you are finished applying the patch, wash your hands with water right away.
When it is time to change your patch, peel off the old patch and apply a new patch to a different skin area.
After you remove your patch, fold it in half with the sticky sides together and flush it down a toilet.
This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Before using fentanyl patches,
tell your doctor and pharmacist if you are allergic to fentanyl, or any other medications, or any of the ingredients in fentanyl patches. Ask your doctor or pharmacist or check the Medication Guide for a list of the ingredients.
tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, and nutritional supplements you are taking or plan to take. Be sure to mention the medications listed in the IMPORTANT WARNING and any of the following medications: antidepressants; antihistamines found in cough, cold, and allergy medications ; buprenorphine Zubsolv ; butorphanol; medications for anxiety; medications for nausea; muscle relaxants; nalbuphine; other medications for pain;pentazocine Talwin ; sedatives; sleeping pills; and tranquilizers. Also tell your doctor or pharmacist if you are taking the following medications or have stopped taking them within the past 2 weeks: monoamine oxidase MAO inhibitors including isocarboxazid Marplan, phenelzine Nardil, selegiline Eldepryl, Emsam, Zelapar, and tranylcypromine Parnate. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
tell your doctor what herbal products you are taking, especially St. John s wort.
tell your doctor if you have or have ever had paralytic ileus condition in which digested food does not move through the intestines. Your doctor may tell you not to use fentanyl patches.
tell your doctor if you have or have ever had seizures; slowed heartbeat; difficulty urinating; low blood pressure; or thyroid, heart, liver, pancreas, gallbladder, or kidney disease.
tell your doctor if you are breast-feeding.
if you are having surgery, including dental surgery, tell the doctor or dentist that you are using fentanyl patches.
you should know that this medication may make you drowsy. Do not drive a car, operate machinery, or do other possibly dangerous activities until you know how this medication affects you.
you should know that fentanyl patches may cause dizziness, lightheadedness, and fainting when you get up too quickly from a lying position. This is more common when you first start using fentanyl patches. To avoid this problem, get out of bed slowly, resting your feet on the floor for a few minutes before standing up.
do not drink alcohol or take prescription or nonprescription medications that contain alcohol while you are using fentanyl patches. Alcohol increases the chance that you will experience serious side effects of this medication.
you should know that fentanyl patches may cause constipation. Talk to your doctor about changing your diet or using other medications to prevent or treat constipation while you are using fentanyl patches.
If you forget to apply or change a fentanyl patch, apply the patch as soon as you remember it. Be sure to remove your used patch before applying a new patch. Wear the new patch for the period of time prescribed by your doctor usually 3 days and then replace it. Do not wear two patches at once unless your doctor has told you that you should.
Fentanyl patches may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
headache
mood changes
feeling cold
drowsiness
depression
confusion
hallucinations seeing things or hearing voices that do not exist
weakness
dizziness
difficulty falling asleep or staying asleep
uncontrollable shaking of a part of the body
pain, burning, tingling, or numbness in the hands or feet
dry mouth
stomach pain
indigestion
diarrhea
nausea
vomiting
loss of appetite
back pain
difficulty urinating
itching
skin irritation, redness, itching, or swelling in the area where you wore the patch
sweating
Some side effects can be serious. If you experience any of these symptoms or those listed in the IMPORTANT WARNING section, call your doctor immediately:
fast or pounding heartbeat
chest pain
seizure
rash
hives
swelling of the eyes, face, mouth, tongue, throat, arms, hands, feet, ankles, or lower legs
hoarseness
difficulty breathing or swallowing
Fentanyl patches may cause other side effects. Call your doctor if you have any unusual problems while you are using fentanyl patches.
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration s FDA MedWatch Adverse Event Reporting program online at or by phone 1-800-332-1088.
Store the fentanyl patches at room temperature and away from excess heat and moisture not in the bathroom.
Throw away any patches that are used, outdated, or no longer needed by carefully removing the adhesive backing, folding the sticky sides of each patch together so that it sticks to itself, and flushing the patches down the toilet. Throw away the pouches and protective liners in the trash. Wash your hands well with water after throwing away fentanyl patches. Do not put unneeded or used fentanyl patches in a garbage can.
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
Symptoms of overdose may include:
difficulty breathing
extreme sleepiness or tiredness
difficulty thinking, talking, or walking normally
small, pinpoint pupils black circles in the center of the eye
faintness
coma loss of consciousness for a period of time
Keep all appointments with your doctor.
This prescription is not refillable. Be sure to schedule appointments with your doctor on a regular basis so that you do not run out of medication if your doctor wants you to continue using fentanyl patches.
It is important for you to keep a written list of all of the prescription and nonprescription over-the-counter medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.

This information may not cover all possible uses, directions, side effects, precautions, allergic reactions, drug interactions, or withdrawal times.
Listen to this article info/dl
Sorry, your browser either has JavaScript disabled or does not have any supported player.
You can download the clip or download a player to play the clip in your browser.
This audio file was created from a revision of the Fentanyl article dated 2011-12-01, and does not reflect subsequent edits to the article. Audio help
More spoken articles
Fentanyl also known as fentanil, brand names Sublimaze, 3 Actiq, Durogesic, Duragesic, Fentora, Matrifen, Haldid, Onsolis, 4 Instanyl, 5 Abstral, 6 Lazanda 7 and others is a potent, synthetic opioid analgesic with a rapid onset and short duration of action. 8 It is a strong agonist at the μ-opioid receptors. Historically it has been used to treat breakthrough pain and is commonly used in pre-procedures as a pain reliever as well as an anesthetic in combination with a benzodiazepine.
Fentanyl is approximately 100 times more potent than morphine, 9 with 100 micrograms of fentanyl approximately equivalent to 10 mg of morphine and 75 mg of pethidine meperidine in analgesic activity. 10 dead link It has an LD50 of 3.1 milligrams per kilogram in rats, and an LD50 of 0.03 milligrams per kilogram in monkeys.
Fentanyl was first synthesized by Paul Janssen in 1960 11 following the medical inception of pethidine several years earlier. Janssen developed fentanyl by assaying analogues of the structurally related drug pethidine for opioid activity. 12 The widespread use of fentanyl triggered the production of fentanyl citrate the salt formed by combining fentanyl and citric acid in a 1:1 stoichiometry, 13 which entered the clinical practice as a general anaesthetic under the trade name Sublimaze in the 1960s. Following this, many other fentanyl analogues were developed and introduced into medical practice, including sufentanil, alfentanil, remifentanil, and lofentanil.
In the mid-1990s, fentanyl was first introduced for widespread palliative use with the clinical introduction of the Duragesic patch, followed in the next decade by the introduction of the first quick-acting prescription formations of fentanyl for personal use, the Actiq lollipop and Fentora buccal tablets. Through the delivery method of transdermal patches, as of 2012 update fentanyl was the most widely used synthetic opioid in clinical practice, citation needed with several new delivery methods currently in development, including a sublingual spray for cancer patients. 14
Fentanyl and derivatives have been used as recreational drugs. Fatalities arising from its use have been recorded.
Medical uses edit
Intravenous fentanyl is extensively used for anesthesia and analgesia, most often in operating rooms, intensive care units and in the prehospital medical setting. The concept of a general anesthetic is based upon a balance between an opioid and a hypnotic agent. Hence, fentanyl is mainly used for induction of anaesthesia alongside a hypnotic agent like propofol. It is also administered in combination with a benzodiazepine, such as midazolam, to produce procedural sedation for endoscopy, cardiac catheterization, oral surgery, etc., and is often used in the management of chronic pain including cancer pain.
Fentanyl transdermal patch Durogesic/Duragesic/Matrifen is used in chronic pain management. The patches work by releasing fentanyl into body fats, which then slowly release the drug into the bloodstream over 48 to 72 hours, allowing for long-lasting relief from pain. The patches are available in generic form and are available for lower costs. Dosage is based on the size of the patch, since the transdermal absorption rate is generally constant at a constant skin temperature.
Rate of absorption is dependent on a number of factors. Body temperature, skin type, amount of body fat, and placement of the patch can have major effects. The different delivery systems used by different makers will also affect individual rates of absorption. The typical patch will take effect under normal circumstances usually within 8–12 hours, thus fentanyl patches are often prescribed with another opiate such as morphine or oxycodone to handle breakthrough pain.
Fentanyl lozenges Actiq are a solid formulation of fentanyl citrate on a stick in the form of a lollipop that dissolves slowly in the mouth for transmucosal absorption. These lozenges are intended for opioid-tolerant individuals and are effective in treating breakthrough cancer pain. It is also useful for breakthrough pain for those suffering bone injuries, severe back pain, neuropathy, arthritis, and some other examples of chronic nonmalignant pain. The unit is a berry-flavored lozenge on a stick which is swabbed on the mucosal surfaces inside the mouth inside of the cheeks, under and on the tongue and gums to release the fentanyl quickly into the system. It is most effective when the lozenge is consumed in 15 minutes. The drug is less effective if swallowed, as despite good absorbance from the small intestine there is extensive first-pass metabolism, leading to an oral bioavailability of 33. These are now available in the United States in generic form, 15 through an FTC consent agreement. 16 However, most patients find it takes 10–15 minutes to use all of one lozenge, and those with a dry mouth cannot use this route. In addition, nurses are unable to document how much of a lozenge has been used by a patient, making drug records inaccurate.
During 2008-09, a wide range of fentanyl preparations became available, including buccal tablets or patches, nasal sprays, inhalers and active transdermal patches heat or electrical. High-quality evidence for their superiority over existing preparations is currently lacking. Some preparations such as nasal sprays and inhalers may result in a rapid response, but the fast onset of high blood levels may compromise safety see below. In addition, the expense of some of these appliances may greatly reduce their cost-effectiveness.
On July 16, 2009, the FDA approved Onsolis BEMA Fentanyl for breakthrough cancer pain. Onsolis incorporates bioerodible mucoadhesive technology, a small soluble film that contains fentanyl which is placed on the inside cheek of the mouth.
In palliative care, transdermal fentanyl has a definite, but limited, role for:
Patients already stabilized on other opioids who have persistent swallowing problem and cannot tolerate other parenteral routes such as subcutaneous administration.
Patients with moderate to severe renal failure.
Troublesome adverse effects on morphine, hydromorphone or oxycodone.
Fentanyl is sometimes given intrathecally as part of spinal anesthesia or epidurally for epidural anesthesia and analgesia. Because of fentanyl s high lipid solubility, its effects are more localized than morphine and some clinicians prefer to use morphine to get a wider spread of analgesia.
Adverse effects edit
Fentanyl s major side effects more than 10 of patients include diarrhea, nausea, constipation, dry mouth, somnolence, confusion, asthenia weakness, and sweating and, less frequently 3 to 10 of patients, abdominal pain, headache, fatigue, anorexia and weight loss, dizziness, nervousness, hallucinations, anxiety, depression, flu-like symptoms, dyspepsia indigestion, dyspnea shortness of breath, hypoventilation, apnea, and urinary retention. Fentanyl use has also been associated with aphasia. 17
Despite being a more potent analgesic, fentanyl tends to induce less nausea, as well as less histamine-mediated itching, in relation to morphine. 18
Like other lipid-soluble drugs, the pharmacodynamics of fentanyl are poorly understood. The manufacturers acknowledge there is no data on the pharmacodynamics of fentanyl in elderly, cachectic or debilitated patients, frequently the type of patient for whom transdermal fentanyl is being used. This may explain the increasing number of reports of respiratory depression events since the late 1970s. 19 20 21 22 23 24 25 In 2006 the U.S. Food and Drug Administration FDA began investigating several respiratory deaths, but doctors in the United Kingdom were not warned of the risks with fentanyl until September 2008. 26 The FDA reported in April 2012 that young children had died or become seriously ill from accidental exposure to a fentanyl skin patch. 27
The precise reason for sudden respiratory depression is unclear, but there are several hypotheses:
Saturation of the body fat compartment in patients with rapid and profound body fat loss patients with cancer, cardiac or infection-induced cachexia can lose 80 of their body fat.
Early carbon dioxide retention causing cutaneous vasodilatation releasing more fentanyl, together with acidosis which reduces protein binding of fentanyl, releasing yet more fentanyl.
Reduced sedation, losing a useful early warning sign of opioid toxicity and resulting in levels closer to respiratory depressant levels.
Fentanyl has a therapeutic index of 270. 28
Overdoses and fatalities edit
A number of fatal fentanyl overdoses have been directly tied to the drug over a period of years. In particular, manufacturers of time-release fentanyl patches have come under scrutiny for defective products. While the fentanyl contained in the patches was safe, a malfunction of the patches caused an excessive amount of fentanyl to leak and become absorbed by patients, resulting in life-threatening side effects and even death. Regardless, Fentanyl is considered the safest opioid medication on the market, as well as the least physically harmful to the body, especially with long-term or life-term use. 29 30
Manufacturers of fentanyl transdermal pain patches have voluntarily recalled numerous lots of their patches, and the U.S. Food and Drug Administration FDA has issued public health advisories related to fentanyl patch dangers. Manufacturers affected include Janssen Pharmaceutica Products, L.P.; Alza Corporation; Actavis South Atlantic, LLC; Sandoz; and Cephalon, Inc. 31
On May 24, 2009, the former guitarist for the band Wilco, Jay Bennett, died in his sleep of an overdose of the drug via Duragesic time-release patches prescribed for him. 32 In 2010, band Slipknot s bassist Paul Gray died after accidentally taking an overdose of a mixture of fentanyl and morphine, for which there was no evidence of a prescription. 33 An inquest jury found by a majority verdict of 3-2 that an overdose of fentanyl was responsible for the death by misadventure of Anita Chan Lai-ling, 69, who died on October 17, 2007, after she was given an overdose of fentanyl. 34
Post-acute withdrawal effects edit
According to a range of medical journals there is also an often under-emphasized potential for post-acute-withdrawal syndrome which may last until after the initial short-term. Post-acute-withdrawal syndrome may induce or mimic psychiatric disorders temporarily or long term such as depression, anxiety disorder, psychosis and in rare cases, even suicidal ideation. Post-acute-withdrawal syndrome will continue for some months usually 1–3 months or more after prolonged cessation of usage which is also referenced in the medical journals on post-acute withdrawal syndrome. citation needed
Chemistry edit
Synthesis edit
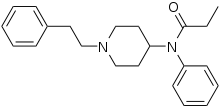
The synthesis of fentanyl by Janssen Pharmaceutica was achieved in four steps, starting from 4-piperidinonehydrochloride. The sequence commenced with N-alkylation of 4-piperidinone with 2-phenylethylbromide to giveN-phenethyl-4-piperidinone NPP. Reductive amination of NPP using aniline and sodium borohydride afforded 4-anilino-N-phenethylpiperidine ANPP. Finally N-acylation of the secondary amine with propionic anhydride provided fentanyl.
Analogues edit
Alfentanil trade name Alfenta, an ultra-short-acting five to 10 minute analgesic.
Sufentanil trade name Sufenta, a potent analgesic five to 10 times more potent than fentanyl for use in specific surgeries and surgery in heavily opioid-tolerant/opioid-dependent patients. Its binding affinity is high enough to theoretically break through a buprenorphine blockade to offer pain relief from acute trauma in patients who are taking high-dose buprenorphine.
Remifentanil trade name Ultiva, currently the shortest-acting opioid, has the benefit of rapid offset, even after prolonged infusions.
Carfentanil trade name Wildnil is an analogue of fentanyl with an analgesic potency 10,000 times that of morphine and is used in veterinary practice to immobilize certain large animals such as elephants.
Lofentanil is an analogue of fentanyl with a potency slightly greater than carfentanil.
3-Methylfentanyl thought to be the active constituent of Kolokol-1, a chemical weapon
3-Methylthiofentanyl
Acetyl-α-methylfentanyl
α-methylfentanyl see below
α-methylthiofentanyl
β-hydroxy-3-methylfentanyl
β-hydroxyfentanyl
p-flurorofentanyl
Thiofentanyl
Mechanism of action edit
Main article: Opioid Pharmacology
Fentanyl provides some of the effects typical of other opioids through its agonism of the opioid receptors. Its strong potency in relation to that of morphine is largely due to its high lipophilicity, per the Meyer-Overton correlation. Because of this, it can more easily penetrate the CNS. 18
Fentanyl binds μ-opioid G-protein-coupled receptors, which inhibit pain neurotransmitter release by decreasing intracellular Ca2 levels.
History edit
Fentanyl was first synthesized by Paul Janssen under the label of his relatively newly formed Janssen Pharmaceutica in 1959. In the 1960s, fentanyl was introduced as an intravenous anesthetic under the trade name of Sublimaze. citation needed In the mid-1990s, Janssen Pharmaceutica developed and introduced into clinical trials the Duragesic patch, which is a formation of an inert alcohol gel infused with select fentanyl doses which are worn to provide constant administration of the opioid over a period of 48 to 72 hours. After a set of successful clinical trials, Duragesic fentanyl patches were introduced into the medical practice.
Following the patch, a flavored lollipop of fentanyl citrate mixed with inert fillers was introduced under the brand name of Actiq, becoming the first quick-acting formation of fentanyl for use with chronic breakthrough pain. More recently, fentanyl has been developed into an effervescent tab for buccal absorption much like the Actiq lollipop, followed by a buccal spray device for fast-acting relief and other delivery methods currently in development.
A fentanyl product has been approved by the US Food and Drug Administration FDA for breakthrough cancer pain called Onsolis. It uses a drug delivery technology called BEMA fentanyl buccal soluble film on a small disc placed in the mouth. Unlike many other fentanyl products, the drug cannot be abused by crushing and inhaling.
Recreational use edit
Fentanyl powder seized by a Lake County Deputy Sheriff in Painesville, Ohio, where a male subject had been discovered unresponsive and struggling to breathe. 35
Illicit use of pharmaceutical fentanyls first appeared in the mid-1970s in the medical community and continues in the present. United States authorities classify fentanyl as a narcotic. To date, more than 12 different analogues of fentanyl have been produced clandestinely and identified in the U.S. drug traffic. The biological effects of the fentanyls are similar to those of heroin, with the exception that many users report a noticeably less euphoric high associated with the drug and stronger sedative and analgesic effects. citation needed
The use of fentanyl has caused death. Because the effects of fentanyl last for only a very short time, regular users may become addicted very quickly. citation needed Additionally, fentanyl may be hundreds of times more potent than street heroin, and tends to produce significantly worse respiratory depression, making it somewhat more dangerous than heroin to users. Fentanyl is most commonly used orally, but like heroin, can also be smoked, snorted or injected. Fentanyl is sometimes sold as heroin, often leading to overdoses. Many fentanyl overdoses are initially classified as heroin overdoses. 36 In Estonia, due to its high rate of recreational use, fentanyl causes more deaths nationwide than traffic accidents. 37
Fentanyl is normally sold on the black market in the form of transdermal fentanyl patches such as Duragesic, diverted from legitimate medical supplies. The patches may be cut up and eaten, or the gel from inside the patch smoked. To prevent the removal of the fentanyl base, Janssen-Cilag, the inventor of the Fentanyl patch, designed the Duragesic patch. The Duragesic patches contain their fentanyl throughout the plastic matrix instead of gel incorporated into a reservoir on the patch. Manufacturers such as Mylan and Sandoz have also produced Duragesic-style fentanyl patches that contain the chemical in a silicone matrix, preventing the removal of the fentanyl-containing gel present in other products. The plastic matrix makes the patches far less suitable to transbuccal use and far more difficult to use illicitly than its gel-filled counterpart. 38
Another dosage form of fentanyl that has appeared on the streets are the Actiq fentanyl lollipops, which are sold under the street name of percopop. The pharmacy retail price ranges from US 15 to US 50 per unit based on strength of lozenge, with the black market cost anywhere from US 20 to US 80 per unit, depending on the strength.
Non-medical use of fentanyl by individuals without opiate tolerance can be very dangerous and has resulted in numerous deaths. 39 Even those with opiate tolerances are at high risk for overdoses. Once the fentanyl is in the user s system it is extremely difficult to stop its course because of the nature of absorption. Illicitly synthesized fentanyl powder has also appeared on the United States market. Because of the extremely high strength of pure fentanyl powder, it is very difficult to dilute appropriately, and often the resulting mixture may be far too strong and, consequently, very dangerous.
Some heroin dealers mix fentanyl powder with heroin to increase potency or compensate for low-quality heroin. In 2006, illegally manufactured, non-pharmaceutical fentanyl often mixed with cocaine or heroin caused an outbreak of overdose deaths in the United States, heavily concentrated in the cities of Dayton, Ohio; Chicago;Detroit; Philadelphia; 40 Baltimore; Pittsburgh; St. Louis; Milwaukee; Camden, New Jersey; 41 Little Rock; and Dallas 42 were also affected. The mixture of fentanyl and heroin is known as magic or the bomb, among other names, on the street. 43
Several large quantities of illicitly produced fentanyl have been seized by U.S. law enforcement agencies. In June 2006, 945 grams of 83 pure fentanyl powder was seized by Border Patrol agents in California from a vehicle which had entered from Mexico. 44 Mexico is the source of much of the illicit fentanyl for sale in the U.S. However, in April 2006 there was one domestic fentanyl lab discovered by law enforcement in Azusa, California. The lab was a source of counterfeit 80-mg OxyContin tablets containing fentanyl instead of oxycodone, as well as bulk fentanyl and other drugs. 45 46
The China White form of fentanyl refers to any of a number of clandestinely produced analogues, especially α-methylfentanyl AMF. 47 This Department of Justice document lists China White as a synonym for a number of fentanyl analogues, including 3-methylfentanyl and α-methylfentanyl, 48 which today are classified as Schedule I drugs in the United States. 49 Part of the motivation for AMF is that despite the extra difficulty from a synthetic standpoint, the resultant drug is relatively more resistant to metabolic degradation. This results in a drug with an increased duration. 50
In June 2013, the United States Centers for Disease Control and Prevention CDC issued a health advisory 51 to emergency departments alerting to 14 overdose deaths among intravenous drug users in Rhode Island associated with acetyl fentanyl, a novel, injected, non-prescription synthetic opioid analog of fentanyl.
Military use edit
Analgesic edit
The Danish Army uses the fentanyl stick in military operations as a painkiller. The war documentary Armadillo 2010 features an interview with a Danish medic who tells of using fentanyl on a severely wounded soldier in Afghanistan. citation needed
United States Air Force Pararescue uses lollipops with fentanyl. 52
As weapon edit
Mossad agents used levofentanyl in their 1997 attempt to kill Hamas leader Khalid Mishal. 53 As noted above, since fentanyl is achiral i.e., has no levo- form, the substance was probably simply fentanyl itself, or perhaps a related or unknown drug.
A gas apparently based on a derivative of fentanyl was used in 2002 as the Moscow hostage crisis chemical agent to incapacitate Chechen terrorist attackers and, unavoidably, their hostages too quickly for them to retaliate. More than 15 of those affected died, including 117 of the 800 hostages. 54
References edit
Janssen Pharmaceuticals Duragesic
Hess R, Stiebler G, Herz A June 1972. Pharmacokinetics of fentanyl in man and the rabbit. Eur. J. Clin. Pharmacol. 4 3 : 137–41. doi:10.1007/BF00561135. PMID 4655287.
FDA Professional Drug Information
Introducing Onsolis. Onsolis.com. Retrieved 2010-07-28.
London, 23 April 2009 PDF. Retrieved 2010-07-28.
Abstral: Prescribing Information. Retrieved 2011-01-07.
Lazanda fentanyl nasal spray CII. Lazanda.com. Retrieved 2012-05-14.
WCPI Focus on Pain Series: The Three Faces of Fentanyl. Aspi.wisc.edu. Retrieved 2010-07-28.
DBL FENTANYL INJECTION. Medsafe. Retrieved 2010-07-28.
Data Sheet. Medsafe.govt.nz. 2008-03-01. Retrieved 2010-07-28.
Stanley TH April 1992. The history and development of the fentanyl series. J Pain Symptom Manage 7 3 Suppl : S3–7. doi:10.1016/0885-3924 92 90047-L. PMID 1517629.
Black J March 2005. A personal perspective on Dr. Paul Janssen. J. Med. Chem. 48 6 : 1687–8. doi:10.1021/jm040195b. PMID 15771410.
DailyMed: About DailyMed. Dailymed.nlm.nih.gov. Retrieved 2010-07-28.
Long Term Safety and Efficacy Study of Fentanyl Sublingual Spray for the Treatment of Breakthrough Cancer Pain - Full Text View. ClinicalTrials.gov. Retrieved 2010-07-28.
Barr Launches Generic ACTIQ R Cancer Pain Management Product Press release. Barr Pharmaceuticals. 27 September 2006. Retrieved 30 September 2006.
With Conditions, FTC Allows Cephalon s Purchase of CIMA, Protecting Competition for Breakthrough Cancer Pain Drugs Press release. FTC. 9 August 2004. Retrieved 30 September 2006.
a b Stacey Mayes, PharmD MS, Marcus Ferrone, PharmD BCNSP, 2006.Fentanyl HCl Patient-Controlled Iontophoretic Transdermal System for Pain: Pharmacology The Annals of Pharmacotherapy
Smydo J 1979. Delayed respiratory depression with fentanyl. Anesth Prog 26 2 : 47–8. PMC 2515983. PMID 295585.
van Leeuwen L, Deen L, Helmers JH August 1981. A comparison of alfentanil and fentanyl in short operations with special reference to their duration of action and postoperative respiratory depression. Anaesthesist 30 8 : 397–9. PMID 6116461.
Brown DL November 1985. Postoperative analgesia following thoracotomy. Danger of delayed respiratory depression. Chest 88 5 : 779–80. doi:10.1378/chest.88.5.779. PMID 4053723.
Bülow HH, Linnemann M, Berg H, Lang-Jensen T, LaCour S, Jonsson T August 1995. Respiratory changes during treatment of postoperative pain with high dose transdermal fentanyl. Acta Anaesthesiol Scand 39 6 : 835–9. doi:10.1111/j.1399-6576.1995.tb04180.x. PMID 7484044.
Nilsson C, Rosberg B June 1982. Recurrence of respiratory depression following neurolept analgesia. Acta Anaesthesiol Scand 26 3 : 240–1. doi:10.1111/j.1399-6576.1982.tb01762.x. PMID 7113633.
McLoughlin R, McQuillan R September 1997. Transdermal fentanyl and respiratory depression. Palliat Med 11 5 : 419. doi:10.1177/026921639701100515. PMID 9472602.
Regnard C, Pelham A December 2003. Severe respiratory depression and sedation with transdermal fentanyl: four case studies. Palliat Med 17 8 : 714–6. PMID 14694924.
Drug Safety Update 2 2 : 2. September 2008 url missing title help.
US FDA: Fentanyl Patch Can Be Deadly to Children
Stanley, Theodore Henry; Petty, William Clayton 1983-03-31. New Anesthetic Agents, Devices, and Monitoring Techniques. Springer. ISBN 978-90-247-2796-4. Retrieved 20 October 2007.
Shapiro, Rick 5 November 2008. Fentanyl Patch Drug Associated with Overdoses Death Claims. InjuryBoard.com. Retrieved 31 August 2009.
Fentanyl Pain Patch Fatal Overdose Risk Causes Watson Patch Recall. BagolieFriedman.com. 13 August 2009. Retrieved 31 August 2009.
Fentanyl Transdermal System marketed as Duragesic and generics. Postmarket Drug Safety Information for Patients and Providers. FDA. 30 April 2009. Retrieved 31 August 2009.
Coroner: Painkiller killed ex-Wilco member. Chicago Tribune. 2009-06-23. Retrieved 2010-08-20.
Slipknot bassist Paul Gray died of morphine overdose. BBC News. 2010-06-21. Retrieved 2010-07-28.
Son vows to sue on painkiller death. 2010-12-03. Retrieved 2010-12-03.
DEA Microgram Bulletin, June 2006. US Drug Enforcement Administration, Office of Forensic Sciences Washington, D.C. 20537. June 2006. Retrieved 22 June 2009.
Boddiger, D. 2006, August 12. Fentanyl-laced street drugs kill hundreds. The Lancet. Retrieved June 15, 2010.
Synthetic drug fentanyl causes overdose boom in Estonia. BBC News. 30 March 2012.
Declaration of Gordon Flynn, Ph.D. PDF. 2004-12-22. Retrieved 2010-07-28.
dead link
CDC MMWR Nonpharmaceutical Fentanyl-Related Deaths - Multiple States, April 2005-March 2007. Cdc.gov. Retrieved 2010-07-28.
Press Release by the Chicago Police Department Police report about a death linked to heroin/fentanyl mixture August 24, 2006
SMU student s death blamed on rare drug. Dallasnews.com. 2006-12-20. Retrieved 2010-07-28.
Fentanyl probe nets 3 suspects by Norman Sinclair and Ronald J. Hansen, The Detroit News, June 23, 2006. Retrieved June 25, 2006.
Intelligence alert: High purity fentanyl seized near Westmoreland, California, DEA Microgram, June 2006
Intelligence alert: Large fentanyl / MDA / TMA laboratory in Azuza, California - possibly the OC-80 tablet source, DEA Microgram, April 2006.
Intelligence alert: Oxycontin mimic tablets containing fentanyl near Atlantic, Iowa, DEA Microgram, January 2006.
List of Schedule I Drugs, U.S. Department of Justice. dead link
Behind the Identification of China White Analytical Chemistry, 53 12, 1379A-1386A 1981
List of Schedule I Drugs, U.S. Department of Justice.
Van Bever WF, Niemegeers CJ, Janssen PA October 1974. Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N- 3-methyl-1- 2-phenylethyl -4-piperidyl -N-phenylpropanamide and N- 3-methyl-1- 1-methyl-2-phenylethyl -4-piperidyl -N-phenylpropanamide. J. Med. Chem. 17 10 : 1047–51. doi:10.1021/jm00256a003. PMID 4420811.
CDC Health Alert Network June 20, 2013. Recommendations for Laboratory Testing for Acetyl Fentanyl and Patient Evaluation and Treatment for Overdose with Synthetic Opioids. Centers for Disease Control and Prevention. Retrieved June 20, 2013.
Shachtman, Noah September 10, 2009. Airborne EMTs Shave Seconds to Save Lives in Afghanistan. Danger Room. Wired.com. Retrieved July 1, 2010.
McGeough, Paul 2009 Kill Khalid - The Failed Mossad Assassination of Khalid Mishal and the Rise of Hamas. Quartet Books. ISBN 978-0-7043-7157-6. Page 184.
Russia names Moscow siege gas. CNN. 2002-10-30.
External links edit
National Institute of Health NIH Medline Plus: Fentanyl Buccal Transmucosal
RxList: Fentanyl
US DEA information: fentanyl
08/16/2007 News Release: Cephalon Announces Positive Results from a Pivotal Study of FENTORA in Opioid-tolerant Patients with Non-cancer Breakthrough Pain
BBC news report on Russian siege story
Lancaster Online story - New Killer: Fentanyl-Heroin Mix
Fentanyl: Emergency Response Database. National Institute for Occupational Safety and Health.
U.S. National Library of Medicine: Drug Information Portal - Fentanyl
Retrieved from Fentanyl oldid 561924382
Categories: General anestheticsSynthetic opioidsPiperidinesAnilidesPropionamidesMu-opioid agonistsJanssen PharmaceuticaBelgian inventionsEuphoriants.


- Easy to read patient leaflet for fentanyl patch. Includes indications, proper use, special instructions, precautions, and possible side effects.
- Fentanyl transdermal patch Durogesic/Duragesic/Matrifen is used in chronic pain management. The patches work by slowly releasing fentanyl through the skin into the.
- Dec 28, 2015 If you forget to apply or change a fentanyl patch, apply the patch as soon as you remember it. Be sure to remove your used patch before applying a new patch.
- INDICATION. DURAGESIC is: A strong prescription pain medicine that contains an opioid narcotic that is used to manage pain severe enough to require daily around.
What Happens If a Fentanyl Patch Is Cut.. Fentanyl is a Schedule II opioid agonist, a powerful narcotic used in the control of recurring moderate to severe chronic pain.